Hemastatx, a spin-off from KU Leuven, has emerged from stealth with its first therapeutic program, HMX-001.
HemastatxTargeting the Mechanism Behind Bleeding
Hemastatx’s lead program is a first-in-class antibody therapy to treat VWF-related bleeding disorders.




Developing targeted therapies for patients with severe bleeding disorders

What is Hemastatx?
Hemastatx is committed to bringing scientific innovation to patients with severe and underserved bleeding disorders, aiming to become a leader in hematology. Founded in 2025 as a spin-off from KU Leuven, Hemastatx builds on over a decade of pioneering research at the Laboratory for Thrombosis Research, led by Prof. Dr. Karen Vanhoorelbeke.
Our first lead program, HMX-001, is a proprietary monoclonal antibody designed to stop bleeding in patients with von Willebrand factor (VWF)-related disorders by targeting the underlying biological mechanism. The program is supported by a robust preclinical data package and broad patent protection.
With headquarters in Switzerland and an R&D hub in Belgium, Hemastatx is strategically positioned within two of Europe’s leading biotech ecosystems.
HMX-001
Lead Program
Mechanism of action
HMX-001 is a first-in-class monoclonal antibody therapy that targets ADAMTS13 — an enzyme that functions like molecular scissors to cleave VWF, a key protein in blood clotting. In patients with VWF-related bleeding disorders, ADAMTS13 can excessively cleave VWF, particularly the most active high-molecular-weight (HMW) multimers, compromising its function. This dysregulation is associated with impaired clot formation and abnormal angiogenesis.
By blocking ADAMTS13 activity and preserving functional HMW VWF, HMX-001 can control severe bleeding episodes. It does so by supporting VWF’s critical biological roles in:
- Hemostasis — enhancing platelet adhesion and promoting clot formation at sites of vascular injury.
- Angiogenesis — reducing bleeding risk associated with abnormal blood vessel formation, such as angiodysplasia.
Differentiation
- First-in-class: The only antibody therapy in development targeting ADAMTS13 for bleeding indications.
- Mechanism-based intervention: HMX-001 restores VWF function by preventing excessive degradation at its source, going beyond downstream symptomatic management.
- Precision targeting: Designed to selectively inhibit ADAMTS13, the primary regulator of VWF function.
Development Stage
Preclinical studies with HMX-001 demonstrate clear target engagement and preservation of HMW VWF multimers across relevant disease models and ex vivo assays. The asset is currently at lead-generation candidate stage. This robust dataset is the base of a broad intellectual property portfolio. Current efforts are focused on strengthening the translational data package and advancing clinical development strategy.

VWF–ADAMTS13 Balance
Understanding the VWF–ADAMTS13 Axis
The VWF–ADAMTS13 interplay under normal hemostasis
VWF is a multimeric glycoprotein that plays a central role in hemostasis by facilitating platelet adhesion at sites of vascular injury and stabilizing coagulation factor VIII. It is synthesized by endothelial cells lining the blood vessels and stored in Weibel-Palade bodies as ultra-large (UL) VWF multimers. These multimers are released through both basal secretion and in response to stimuli such as vascular injury and inflammation.
Once released, UL-VWF multimers undergo shear-dependent unfolding, transitioning from a compact, globular form to an elongated conformation. This structural change exposes the ADAMTS13 cleavage site.
ADAMTS13, a plasma metalloprotease, is the primary regulator of VWF activity. It specifically cleaves these partially unfolded UL-VWF multimers into smaller, functionally active high-molecular-weight (HMW) multimers.
VWF’s hemostatic potency is directly linked to its size: HMW multimers are significantly more effective at anchoring platelets to damaged vessel walls. Following vascular injury, these HMW multimers rapidly recruit platelets, forming a stable VWF–platelet plug that prevents excessive blood loss.
Impaired hemostasis by excessive VWF cleavage
Under pathological conditions—such as increased shear stress or mutations in VWF—abnormal unfolding can lead to enhanced exposure of the ADAMTS13 cleavage site. This dysregulation of the VWF–ADAMTS13 axis may result in excessive cleavage of HMW multimers, reducing the availability of functionally active VWF.
The resulting loss of HMW multimers impairs platelet adhesion and compromises hemostatic function, resulting in VWF-related bleeding disorders.
Beyond its hemostatic role, VWF also contributes to vascular integrity. Experimental evidence suggests that VWF deficiency may promote aberrant angiogenesis. Clinically, patients with VWF-related disorders frequently present with gastrointestinal bleeding originating from vascular malformations such as angiodysplasia.
Pathophysiology & indications
One Mechanism. Multiple Patient Populations.
In both acquired and genetic conditions, pathological unfolding of VWF exposes it to excessive cleavage by ADAMTS13, leading to the loss of HMW VWF multimers and impaired clotting.
Acquired von Willebrand Syndrome (aVWS):
Occurs in patients with advanced cardiovascular disease, including those receiving mechanical circulatory support:
- Left ventricular assist devices (LVADs) provide continuous blood flow from the left ventricle to the aorta in patients with chronic heart failure.
- Impella® devices offer temporary cardiac support to maintain perfusion during acute or high-risk procedures.
- Extracorporeal membrane oxygenation (ECMO) delivers short-term heart-lung support by circulating blood through an external oxygenator.
All of these devices expose blood to high shear forces, promoting pathological VWF unfolding and excessive degradation by ADAMTS13.
Severe aortic stenosis similarly induces elevated shear stress. In Heyde syndrome, this is frequently associated with gastrointestinal bleeding due to angiodysplasia.
Congenital von Willebrand Disease (VWD Type 2A):
- Caused by Group II mutations in the VWF gene that make the protein more susceptible to ADAMTS13 cleavage, even under physiological flow conditions.

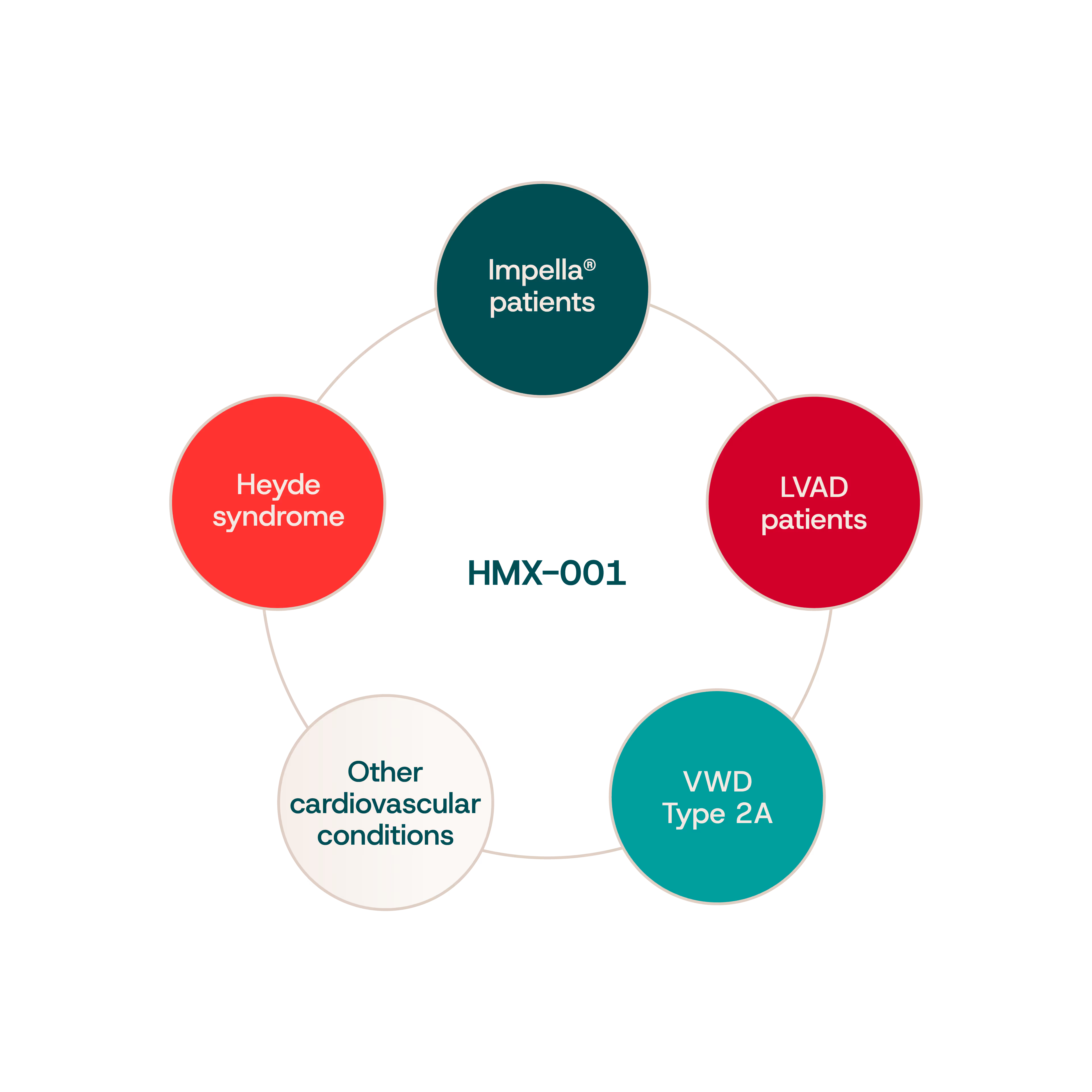
Pipeline-in-a-product opportunity
By addressing this common biological mechanism, HMX-001 unlocks therapeutic potential across diverse patient populations with unmet clinical needs.
This highlights HMX-001 as a pipeline-in-a-product market opportunity; a single therapy with the potential to treat both acquired and inherited forms of VWF-related bleeding disorders.
Unmet Need & Patient Burden
Patients affected by VWF-related bleeding disorders experience significant health challenges and reduced quality of life.
- Impaired clotting leads to spontaneous mucosal bleeding (e.g., nosebleeds, gastrointestinal bleeding) and prolonged bleeding after injury or surgery.
- Chronic VWF deficiency promotes the development of angiodysplasia (fragile, abnormally formed blood vessels in the gastrointestinal tract), which further exacerbates gastrointestinal bleeding.
These patients often suffer from recurrent bleeding episodes, severe anemia, fatigue, and frequent hospitalizations. Many rely on blood transfusions and undergo invasive procedures such as colonoscopies. Despite these burdens, targeted treatments that address the underlying mechanism of disease remain lacking.

Scientific Publications
HMX-001 is built on over a decade of academic research into the role of VWF and ADAMTS13 in bleeding pathophysiology. The foundational work at KU Leuven has provided key insights into how their imbalance drives clinical complications — and how restoring this balance with an ADAMTS13 antibody offers a new therapeutic avenue.
The people behind Hemastatx
The Hemastatx team brings together deep expertise in hemostasis, target biology, antibody development, and venture building.

Kevin Hollevoet, Phd
CEO & Co-founder
Kevin Hollevoet, PhD, is a seasoned biotech executive and venture builder, passionate about advancing therapeutic innovations, including single assets and platform technologies. He has held various roles in biotech companies and operates in life sciences hubs across Western Europe, the Nordics, and the United States. Dr. Hollevoet holds a PhD from Ghent University and completed postdoctoral training at the US National Cancer Institute and KU Leuven. He has strengthened his strategic and business acumen through postgraduate and executive business programs at KU Leuven and Vlerick Business School.

Shannen Deconinck, PhD
Preclinical R&D Lead & Co-founder
Shannen Deconinck, PhD, is an expert on ADAMTS13 targeting to restore hemostasis. Her PhD and postdoctoral research at KU Leuven Kulak focused on developing an ADAMTS13 antibody therapy to treat bleeding after left ventricular assist device implantation in heart failure patients. Dr. Deconinck’s work has been recognized with the prestigious KU Leuven Da Vinci award. She holds two patents related to this innovative work.

Prof. Karen Vanhoorelbeke
Advisor & Co-founder
Prof. Karen Vanhoorelbeke, PhD, is an internationally renowned expert in the role of VWF and ADAMTS13 in hemostasis and thrombosis. She serves as Principal Investigator of the Laboratory for Thrombosis Research at KU Leuven Kulak and is co-founder and head of PharmAbs, the KU Leuven Antibody Center. Prof. Vanhoorelbeke coordinates several European projects, reflecting her large international network with academia and industry. In 2022, she received the ISTH Esteemed Career Award. Her track record includes over 200 peer-reviewed publications, four patents and a diagnostic product on the market.

Nick Geukens, PhD
Advisor & Co-founder
Nick Geukens, PhD, is the General and Innovation Manager of PharmAbs, the KU Leuven Antibody Center. He is a trained Biochemist and attended several innovation and entrepreneurship programs at Flanders Business School. He brings extensive expertise in antibody discovery and translational research. Under his leadership, PharmAbs has out-licensed ten diagnostic tests to the market, and multiple antibody-based therapy products are being developed in-house. Dr. Geukens has 76 peer-reviewed publications and is co-inventor of five patents.

Olivier Léger, PhD
Advisor Antibody Engineering & Development, independent consultant
Olivier Léger, PhD, is an experienced antibody engineer and biotech consultant with over 30 years of experience driving therapeutic innovation from discovery through development. He has contributed to several approved monoclonal antibodies, including emapalumab, avelumab, and natalizumab, and has helped advance novel bispecific and nanobody-based assets. Dr. Léger is a co-founder of antibody-focused ventures such as Deeptope and Kekkan Biologics, and holds 40 patents alongside numerous scientific publications. His expertise spans antibody engineering, affinity maturation, IP strategy, and early-stage drug development.

Hanspeter Rottensteiner, PhD
Advisor Preclinical Development, independent consultant
Hanspeter Rottensteiner, PhD, is a seasoned Research & Development Executive with a passion to drive biopharmaceutical innovation. With deep expertise in drug discovery and development, he has successfully advanced early-stage therapeutics to the clinic and forged strategic partnerships across academia and industry. He has played a pivotal role in the development of several approved enzyme replacement therapies, including recombinant ADAMTS13 and von Willebrand factor. His expertise spans biologics, gene therapy, IP strategy and translational science.

Our Investors
We have secured initial funding from an international syndicate of early-stage investors, led by BaseLaunch and joined by Gemma Frisius Fund and the Butterfly Fund.
Latest Updates & Events
Let’s shape the future of Hemastatx together
Interested in collaborating, investing, or learning more about Hemastatx? We’d love to hear from you.














